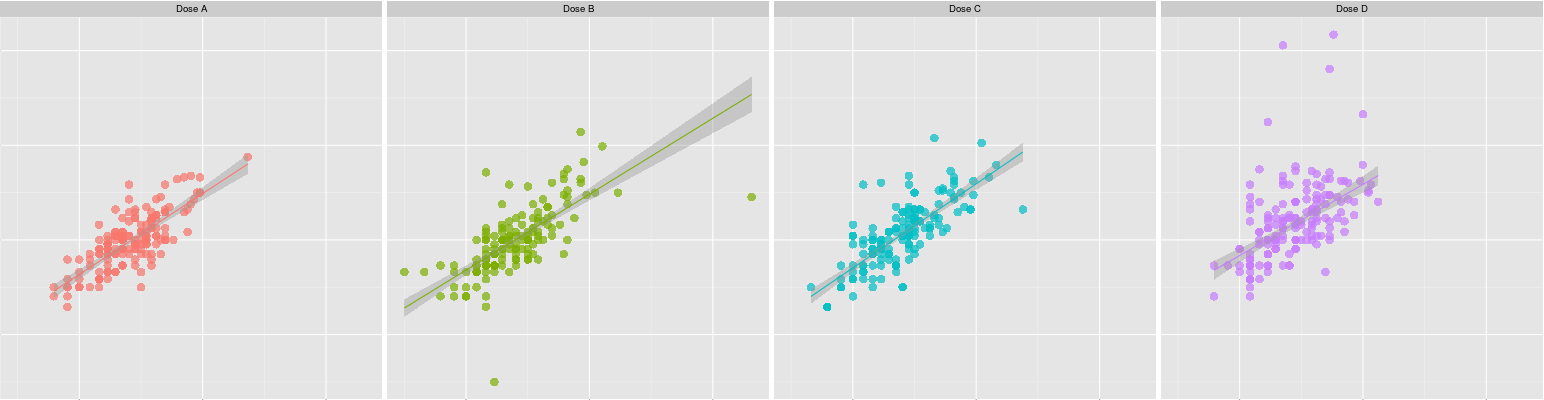
Liver enzyme elevations and a dose-response relationship
A small phase 2 study in a novel compound raised questions about elevated
liver enzymes. The number of patients affected was small, and some of the
elevations were not considered clinically important, but the relationship to
dose and demographic factors was unclear.
We helped the client understand the data by performing extreme value
modelling. We found clear evidence of a
dose-response relationship. Moreover, the patients most affected were those who
most closely resembled the anticipated target patient population.
Simulations from the fitted model suggested that an unacceptable proportion of
patients would be likely to have severe liver enzyme elevations if larger numbers
of patients were exposed at therapeutic doses. The company was able to terminate
development, avoiding the estimated $1M cost of their next planned clinical
trial, and move on to other opportunities.
Understanding unexpected safety findings from a phase 2 study
Scientists at a biotech company running a phase 2 study of a novel treatment
noticed a few unexpected adverse events in their highest dose group.
We enabled the client to gain a better understanding of the issue by
developing novel graphical representations, showing
all the events of interest, their time of onset, and a lab variable thought
to be implicated. The graphics were made
interactive, enabling easy browsing and allowing drilling down into the data.
The company was able to present their understanding of the data to prospective
partners in the knowledge that they had deep and detailed knowledge of the issues
and that they could honestly portray the drug's risk profile across the dose range.
Identifying subgroups of responders
A small number of patients in a phase 3 programme had elevations
in a clinical biomarker. Whilst the efficacy of the drug was
established in the overall patient population, the significance of the
elevations was unknown.
We undertook a number of activities to help the client gain a thorough
understanding of the data. Firstly, we provided
Mercury
to enable clinical reviewers to clearly see population-level effects and to
quickly drill down to patient-level data. We then performed state of
the art data mining to reduce hundreds of potential
predictors of biomarker elevation to a shorlist of 5. The predictors in this
shortlist were then subjected to thorough statistical analysis and visualization.
Whilst we were able to identify a subgroup of patients who were predisposed
to biomarker elevation, the risk/benefit
ratio was still favourable in the subgroup. With
this context, the client successfully made the case for marketing
authorization in the population as a whole, rather than in a restricted
subgroup.
Modelling individual patients given minimal information
A client was developing a new compound for treatment of conditions in which
patients move into and out of a state of disease remission over time. Various
study designs and sizes were being considered, but there was great uncertainty
about the required treatment effect.
We helped the client by developing an approach to simulating
many clinical studies of such patients, using summary data in the published
literature. We used the simulated studies to identify
a novel approach to the analysis of the data resulting in much lower sample
size requirements.
Besides a report of our conclusions and recommendations, we provided
software and documentation to enable the client to run their own simulations
and size their own studies in the future.
|